 |
The News Service | |||
News | ||||
|
Prions Rapidly “Remodel” Good Protein Into Bad, Brown Study Shows
Brown Medical School researchers have discovered that prions – the culprits behind fatal brain diseases such as mad cow and its human counterparts – convert healthy protein into abnormal protein through an ultrafast process similar to DNA replication. The breakthrough finding, published in Nature, helps explain how prions multiply and lead to illness. PROVIDENCE, R.I. — Two Brown Medical School biologists have figured out the fate of healthy protein when it comes in contact with the infectious prion form in yeast: The protein converts to the prion form, rendering it infectious. In an instant, good protein goes bad.  When good proteins go bad This quick-change “mating” maneuver sheds important light on the mysterious molecular machinery behind prions, infectious proteins that cause fatal brain ailments such as mad cow disease and scrapie in animals and, in rare cases, Creutzfeldt-Jacob disease and kuru in humans. Because similar protein self-replication occurs in neurodegenerative diseases, the findings, published in the latest issue of Nature, may also help explain the progression of Alzheimer’s, Parkinson’s and Huntington’s diseases. Graduate student Prasanna Satpute-Krishnan and Assistant Professor Tricia Serio, both in Brown’s Department of Molecular Biology, Cell Biology and Biochemistry, conducted the research using Sup35, a yeast protein similar to the human prion protein PrP. The researchers tagged a non-prion form of Sup35 with green fluorescent protein in one group of cells and “mated” these cells with another group that contained the prion form. When the two forms came in contact in the same cell, the green-glowing, healthy protein changed pattern – a visual sign that it converted to the prion form. These results were confirmed in a series of experiments using different biochemical and genetic techniques. 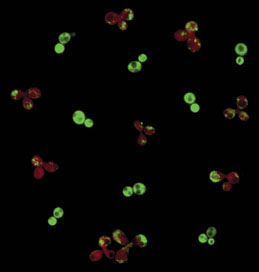 Green light/red light Because proteins can’t replicate like DNA and RNA – the genetic material in bacteria, viruses and other infectious agents – the research helps explain the puzzling process of how prions multiply and spread infection. Satpute-Krishnan said the speed of protein conversion was surprising. “The prions were taking all the existing protein and refolding it immediately,” she said. “It’s a very, very rapid change.” After the conversion, the yeast cells remained healthy but had new characteristics. This survival supports the theory that prions have endured through evolution because shape-shifting is advantageous, allowing cells to avoid stress by rapidly adjusting to a new environment. “Our studies provide some insight into how the appearance of a misfolded protein – a rare event – can lead to devastating neurological diseases,” said Serio. “Just a small amount of prion-state protein can rapidly convert healthy protein into a pathogenic form.” The National Cancer Institute and the Pew Scholars Program in the Biomedical Sciences funded the research. ###### News Service Home | Top of File | e-Subscribe | Brown Home Page | ||||